Clinical trials in Republic of Belarus
Belarus. Country Overview
Belarus has much to offer pharmaceutical companies as a location for clinical trials. Belarus, officially the Republic of Belarus, is a country in Eastern Europe. Belarus is the 13th largest and 20th most populous country in Europe, with a population of 9.2 million. With such a large population, Belarus is not saturated with competing trials, which can be an opportunity for clinical trial sponsors.
The medical care provided by doctors and hospitals in Belarus is above average and exceeds the average achieved in EU countries. The country has 10.8 hospital beds per 1,000 inhabitants. The global average is 2.9 beds. Within the EU, there are 4.6 beds per 1,000 inhabitants. Overall, the density of physicians in Belarus has increased over the period. There are approximately 41 physicians per 10,000 inhabitants in Belarus.
Physicians in Belarus are both highly trained and motivated to act as Principal Investigators (PIs). They speak English at an intermediate or advanced level and are trained in GCP.
Belarus is particularly well suited for trials in oncology, cardiology, rheumatology, and transplantation, as Minsk is home to large republican scientific centers specializing in these areas. These centers are well-equipped, staffed by clinicians experienced in conducting clinical trials, and have access to an extensive patient database of potential trial participants.
Belarus provides universal healthcare. This means that most of the population has access to many free healthcare services. The Belarusian Ministry of Health is working to improve the availability and quality of medical care for all citizens of the country without exception, primarily through outpatient healthcare facilities. Belarus remains committed to the principle of universal access to health care, which is provided free at the point of use through predominantly state-owned facilities organized hierarchically on a territorial basis. Healthcare is provided in regional clinics and republican centers in urban areas and through outpatient clinics in rural areas. Rural patients are referred to urban centers for further consultation and treatment as necessary. In most cases, patients must be referred to specialists by a general practitioner after an examination. This means that the flow of patients in Belarus is predictable and patients are concentrated in large hospitals which is good for clinical trials.
Pharmaceutical coverage is generally limited to the cheapest products - typically local drugs or cheap generics. This situation contributes to the high percentage of patients who have not been exposed to the latest pharmaceutical advances and who are eager to gain access to breakthrough treatments offered through clinical trials. They recognize that the process provides them with more intensive medical care than would otherwise be available. Trial participants have access to the latest advances in treatment (rather than local generics) and benefit from additional examinations and tests under the constant supervision of the PI.
The country has a high percentage of patients who have not been exposed to modern treatments; it is a country where drug-naive patients can be identified.
The urban population of Belarus is about 79.91 per cent. The largest cities are Minsk, Gomel, Mogilev and Vitebsk. It is not necessary to initiate study sites in many cities to enroll patients in Belarus.
According to the World Bank, the literacy rate among adults and youth in Belarus reached 99.9% in 2019. This means that the ICF process is well understood by patients.
The cost of living in Belarus is comparatively low. The average monthly salary in Minsk is $500. This translates into significant savings for clinical trial sponsors.
The clinical trials landscape in Belarus
According to clinicaltrials.gov, there were 333 trials in Belarus, of which 90 were active as of 04/04/2013.
According to the Belarusian Clinical Trials Registry, there were 1377 trials since 2001. The distribution by phase is as follows:
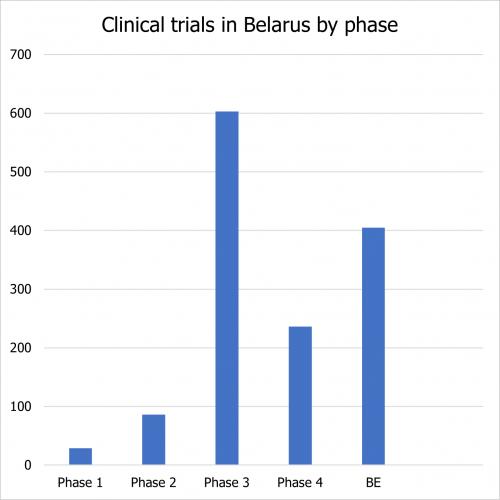
530 trials are national trials conducted by Belarusian pharmaceutical companies. 417 are clinical trials conducted in Belarus by international companies. 430 are multicenter trials.
Regulatory landscape in Belarus
The key document for the conduct of clinical trials in the Republic of Belarus is the Regulation of the Ministry of Health of the Republic of Belarus on Clinical Trials of Medicinal Products from 6 November 2020 # 94.
A clinical trial is approved by a decision of the Ministry of Health, which is preceded by a set of preliminary technical works related to expert examinations carried out by the Republican Unitary Enterprise "Centre of Expertise and Testing in Healthcare"; approval of the clinical trial by an independent ethics committee of the healthcare organization, on the basis of which the clinical trial is planned. The total duration of the preparatory work may not exceed 120 calendar days. This period may be extended to 360 calendar days by agreement between the parties. Based on the results of the preliminary examination, the Centre issues a conclusion on the presence (absence) of grounds for the Ministry of Health to issue a permit to conduct a clinical trial. The conclusion is then submitted to the Ethics Committee, and the Ethics Committee of the health care organization conducts an expert evaluation of the submitted documents, materials and information, as well as an evaluation of the investigators' qualifications and the availability of conditions in the health care organization for conducting clinical trials in accordance with the requirements of Good Clinical Practice on the basis of the agreement concluded between the applicant and the health care organization. The expert evaluation and issuance of a conclusion on approval to conduct a clinical trial by the Ethics Committee of a health care organization shall be carried out in accordance with the procedure and conditions established by the Regulation on the Independent Ethics Committee, approved by the resolution on the adoption of these Regulations.
In addition, to obtain approval to conduct a clinical trial, the applicant shall apply to the Ministry of Health after preliminary examination and expert evaluation. The Centre shall, with the written consent of the applicant, provide the Ministry of Health with access to the documents previously submitted by the applicant to the Centre for a series of preliminary works. The Ministry of Health makes its decision based on the results of the review of the application.
After obtaining approval to conduct a clinical trial, the sponsor concludes contracts with health care organizations specified in the approval of the Ministry of Health and the clinical trial protocol.
Main documents to submit to the MoH in Belarus to initiate a clinical trial:
- Study protocol
- Investigator's brochure
- Preclinical study reports to the extent required by the Good Laboratory Practice Rules of the Eurasian Economic Union in the field of drug circulation
- Individual registration card (except for multicenter clinical trials)
- Document issued to a contract research organisation with clearly delegated responsibilities (in case CRO is an applicant)
- Written information for study subject and informed consent form
- Other written information for the study subject
- Example of labeling with information about a medicinal product in Russian or Belarusian
- Appropriate approvals that apply for clinical trials or medicines with special characteristics (if any), e.g. genetically modified organisms, radiopharmaceuticals
- Copy of the veterinary certificate (manufacturer's declaration) stating that the source components of animal origin potentially infecting spongiform encephalopathies (if applicable) have not been used in the production
- Quality certificates of the medicinal products
- A copy of the manufacturing licence, indicating the scope of the licence
- Information on the manufacturer of the investigational drug
- Information on the manufacturing (production) technology of the investigational medicinal product and documentation that monitors the manufacturing process and quality control of the medicinal product
- Written confirmation that work at the manufacturing site is performed in accordance with Good Manufacturing Practice requirements
Clinical trials of medicinal products are conducted only in health care organizations designated by the Ministry of Health.
As Belarus is a member of the EAEU, it follows the EAEU guidelines for clinical trials. Clinical trials for EAEU registration are conducted in accordance with EAEU requirements. It is also important to note that registration of medicines in the EAEU may require patients to be enrolled in one of the member countries of the Union. Other countries in the EAEU are Russia, Armenia, Kazakhstan, Armenia, Kyrgyzstan.
Clinical trial quality in Belarus
In Belarus there were 2 FDA inspections. Both inspections resulted in NAI. No Action Indicated (NAI) means no objectionable conditions or practices were found during the inspection (or the objectionable conditions found do not justify further regulatory action). This can mean that that clinical trials in Belarus are conducted at a high level of quality.
Summary. Clinical trials in Belarus
Belarus is a hidden gem when it comes to clinical trials. There are some advantages that the country can offer:
- High enrolment rates due to a highly motivated patient population, three-quarters of whom live in urban centers (a ratio similar to France and Germany).
- A moderately large, drug-naïve population open to modern treatment.
- A large pool of enthusiastic, English-speaking physicians whose experience in clinical research is constantly evolving.
- A highly educated and well-trained medical staff who are familiar with Good Clinical Practices (GCP) (the regulatory standard) and who meet the requirements of both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
- An overall cost structure that is favorable compared to other regions.
- Belarus has the clinical infrastructure to ensure patient safety, quality data and efficient operations.
- Transparent regulatory requirements
Smooth drug development - a CRO in Belarus.
As a trusted biopharmaceutical partner, we have full-service research capabilities in high recruitment countries. We provide full service clinical trials in Europe, CIS, EAEU and India. We ensure client satisfaction through fast, efficient and cost-effective enrollment of patients. Smooth Drug Development has a presence in Belarus. We are able to conduct clinical trials in Belarus or include Belarusian sites into global trials.
