Clinical trials in Turkey
Clinical trial landscape in Turkey
According to clinicaltrials.gov, the number of clinical trials, including observational and interventional studies, funded by industry, sponsors and academia is steadily increasing in Turkey. Since 2011, there has been a steady annual increase in the number of clinical trials in Turkey. In the first quarter of 2023, the number of trials already reached the number of 967 trials.
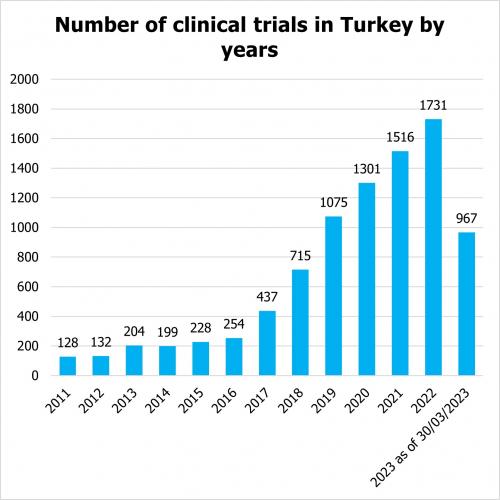
According to clinicaltrials.gov there are 2, 699 clinical trials which are registered as active (not yet enrolling, enrolling, enrolling by invitation, active, not recruiting) as of 2023. Out of them there are 750 interventional studies that are industry sponsored trials. The distribution across phases of clinical trials in Turkey is as follows:
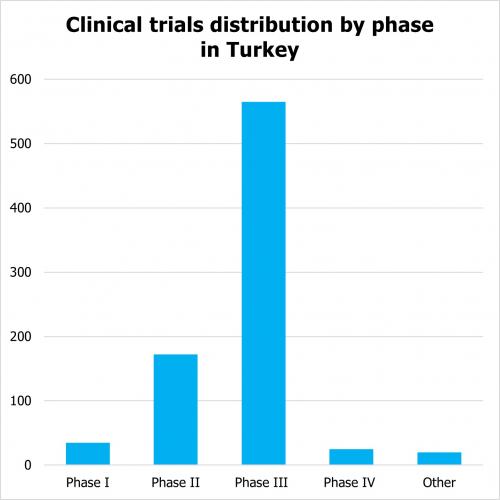
Clinical trials conducted in Turkey cover all major therapeutic areas. Clinical trials in Turkey also include pediatric trials. For example, as of 30/03/2023 there were 83 pediatric trials in Turkey.
Turkey has ample room for growth to increase its share of global clinical research, given its large population.
Turkey can further improve and become a leader in the broader region of the Middle East and Central and Eastern Europe to become a top 10 global clinical research country.
Regulatory landscape for clinical trials in Turkey
Clinical trial applications for both pharmaceuticals and medical devices are submitted to the Turkish Pharmaceuticals and Medical Devices Agency (TİTCK).
Clinical trials must be conducted at sites dedicated to clinical research with appropriate staff, equipment, and laboratories.
For clinical trial applications related to pharmaceuticals and medical devices, the sponsor of the clinical trial must first apply to TİTCK and the Ethics Committee. TİTCK and EC approvals are granted in parallel. TİTCK and EC approval can be obtained in 4-6 weeks if no questions are raised.
Most documents can be submitted in the English language. Patient-related documentation, insurance and protocol synopsis must be submitted in Turkish. It is not necessary to submit an IMPD or go through the QP release process to initiate a clinical trial in Turkey.
The sponsor may also appoint a Contract Research Organisation (CRO) based in Turkey to submit the application.
If the sponsor does not have a representative in Turkey, the clinical trial application must be submitted through a CRO located in Turkey.
Import and export licenses are required to import the investigational product into Turkey and to export samples out of Turkey.
Quality of clinical trials in Turkey
There were 4 FDA clinical Investigator Inspections in Turkey according to www.fda.gov/. The findings were 3 NAI (No Action Indicated) and 1 VAI (voluntary action indicated).
Summary. Clinical trials in Turkey
The country has built a great infrastructure for clinical trials, which is why many sponsors choose Turkey for their next clinical trials. The advantages of performing clinical trials in Tukey in brief are:
- Big population;
- Large number of sites and investigators;
- Developed clinical trials infrastructure;
- Higher percentage of treatment-naive patients interested in participating in clinical trials and easy access to them compared to Western Europe and the USA;
- Full compliance with GCP and quality standards;
- Low number of studies per patient;
- Moderate clinical research costs and investigator fees;
- Concentration of sites in major cities;
- EU QP statement is not required to initiate a trial in Turkey;
- No IMPD required to initiate a trial in Turkey;
- No need to translate clinical trial documents into Turkish.
Smooth Drug Development — a CRO in Turkey
Smooth Drug Development is a full service CRO in EAEU, CIS, EC, Middle East and India. Smooth Drug Development has an experienced local team to effectively manage clinical trials in Turkey. Our experienced team in Turkey delivers exceptional data quality, reliable results within study budget.
Smooth Drug Development has in-depth local experience and knowledge of the region which translates into rapid regulatory approval, rapid site contracting and exceptional patient recruitment in Turkey.
If you are considering conducting clinical trials in Turkey or an international study with clinical research sites in Turkey, our team will be happy to answer any questions you may have about clinical trials in Turkey.
Smooth Drug Development partners with leading clinical research sites and Site Management Organizations in Turkey.
Smooth Drug Development has capabilities to provide full spectrum of clinical trials services for phase I-IV trials and observational studies in Turkey:
- Project management;
- Regulatory support;
- Medical writing;
- Biomedical statistics;
- Study feasibility;
- Data management / IWRS;
- ePRO;
- eTMF/eISF service;
- Logistics and storage;
- Clinical monitoring;
- Centralized monitoring;
- Medical monitoring;
- Pharmacovigilance;
- Central laboratory;
- Patient logistics;
- Clinical study report.
