Clinical trials in Republic of Uzbekistan
Uzbekistan. Country Overview
Although the density of clinical trials in Uzbekistan is comparatively low, Uzbekistan could be a good place for clinical trials.
Uzbekistan is a country located in Central Asia.
Uzbekistan is the most populous country in Central Asia. Its 36 million citizens make up almost half of the region's total population. Other countries in the region are Kazakhstan (population: 16.6 million), Kyrgyzstan (population: 5.6 million), Tajikistan (population: 8.4 million) and Turkmenistan (population: 5.3 million).
Uzbekistan's population is currently growing at a rate of 1.48% per year.
Uzbekistan is the 41st largest country in the world and the third largest of the former Soviet states after Russia (145 million) and Ukraine (41 million). Given its large population, Uzbekistan could be a great place to conduct clinical trials.
The demographics of Uzbekistan are 65% Caucasian and 35% Asian.
The largest city in Uzbekistan is the capital, Tashkent. Other major cities in Uzbekistan are Namangan, Samarkand and Andijan. Uzbekistan's population is densest in the east of the country, around the major cities. The proportion of Uzbekistan's population living in urban areas is around 50.43 per cent. Due to the country's urbanization, it is convenient to conduct clinical trials in the country's largest cities.
Residents of Uzbekistan have free access to medical services provided by a network of state medical institutions (outpatient clinics), first aid stations and state hospitals. The healthcare system has a very transparent flow of patients from outpatient clinics to specialized healthcare centers, which is very good for clinical trials because patients are concentrated in large hospitals.
Although the entire population of the country is covered by health insurance, the level of health care is quite low and still needs to be improved, which makes Uzbekistan the right place for some specific trials, for example trials that require drug-naive patients or patients with specific diseases. For example, the most common diseases are those associated with contaminated drinking water: typhoid, hepatitis, dysentery, cholera and various types of cancer. The main causes of death are, in order of frequency, diseases of the cardiovascular, respiratory and digestive systems, and infectious and parasitic diseases. The incidence of tuberculosis, AIDS/HIV and brucellosis is high in Uzbekistan.
Based on our experience in Uzbekistan, the patient population is receptive to participating in clinical trials to gain access to new treatments, as the choice of treatments available in Uzbekistan is limited.
In 2021 the number of hospitals of all types operating in the republic is 1281, including 637 private hospitals.
As of 1 January 2020, the number of doctors in the Republic of Uzbekistan is 91.9 thousand. The provision with doctors per 10 000 inhabitants is 27,1.
The number of hospital beds per 1000 inhabitants is 4.
The cost of living in Uzbekistan is relatively low. It is ranked 127 out of 140. On 1 March 2023, the average salary in Uzbekistan is USD 350. This brings some savings to trial sponsors as trials can be conducted at a lower cost compared to western countries and even compared to some emerging clinical trial regions. Site and PI grants will definitely be lower in Uzbekistan.
Clinical trials landscape in Uzbekistan
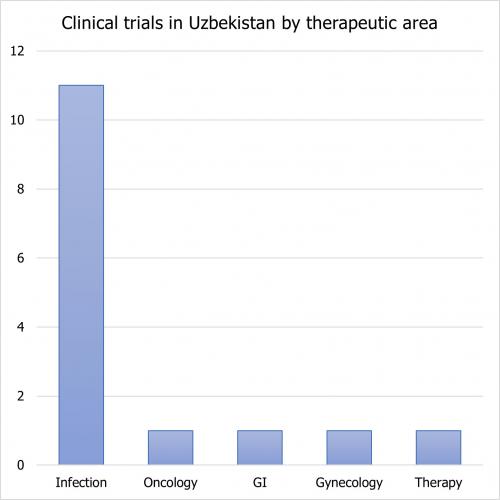
The number of trials conducted in Uzbekistan according to clinicaltrials.gov is low, with only 37 trials. However, this number is likely to be higher, as companies from the EAEU conduct a number of trials in the country that are not published on the clinicaltrials.gov website. The majority of clinical trials in Uzbekistan are in infectious and microbial diseases.
According to clinicaltrials.gov, there were 17 interventional trials.
The majority of trials were in infectious diseases:
- COVID-19
- Tonsillitis
- Bronchitis
- Tuberculosis
- Flu
- Diarrhea
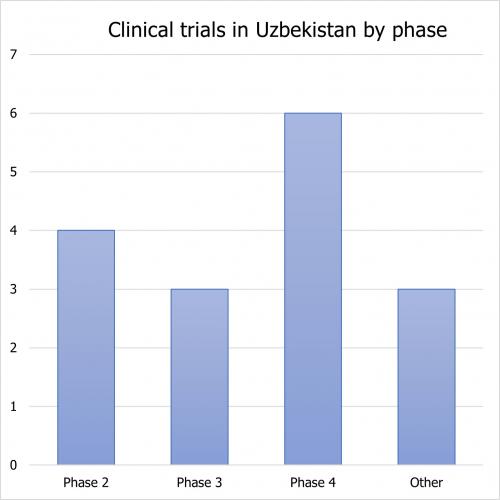
The distribution by phase is as follows:
The country has the potential to attract new clinical trial projects. Uzbekistan is definitely an emerging region for clinical research. In addition, we are seeing a lot of interest from Western companies in the country as new clinical trial markets are needed due to high competition and time and budget pressures.
In addition, the government of Uzbekistan is taking initiatives in the area of clinical trials. For example, the Cabinet of Ministers has adopted a resolution on organizational measures for the development of clinical trials of medicines and their implementation in accordance with the requirements of international standards.
No FDA inspections were performed in Uzbekistan.
Regulatory environment for clinical trials in Uzbekistan
The Ministry of Health of the Republic of Uzbekistan ("the Ministry of Health") is the central government body for health care, which participates in the development and implementation of government programs in the field of medicines and pharmaceutical activities, and is responsible for licensing pharmaceutical activities and state registration and quality control of medical devices and equipment.
The State Centre for Testing and Standardization of Medicines, Medical Devices and Medical Equipment Agencies for the Development of the Pharmaceutical Industry under the Ministry of Health of the Republic of Uzbekistan ("the State Centre") is a working body specializing in state registration, quality control, standardisation and certification of medicines, medical devices and medical equipment. The Pharmacological Committee is a subdivision of the State Centre.
The procedure for conducting clinical trials is primarily regulated by the Regulation on Clinical Trials. According to Decree No. 213 and the Regulation on Clinical Trials, the Pharmacological Committee is an authorized state body for the conduct and control of clinical trials. Therefore, the decision to conduct clinical trials of pharmacological products and drugs is made by the Pharmacological Committee.
Clinical trial materials must be reviewed by the Pharmacological Committee and the Ethics Committee.
Based on Smooth Drug Development's experience, the timelines for study approvals are approximately 2 months.
Summary. Clinical trials in Uzbekistan
Although Uzbekistan does not have a long history of clinical trials, the country may be interesting for conducting clinical trials for the following reasons:
- Large population;
- No competition for trial patients;
- Access to untreated patients;
- Specific studies can be conducted in the country, such as infectious and bacterial diseases;
- Fast approval of 2 months;
- Cost-effectiveness compared to Western countries and even some emerging clinical trial regions.
Smooth Drug Development — a CRO in Uzbekistan
Smooth Drug Development is a full service CRO operating in Europe, EAEU and Asia. Smooth Drug Development has experience in conducting clinical trials in Uzbekistan. We can conduct a local trial in Uzbekistan as well as a global trial with sites in Uzbekistan. We have a local team in Uzbekistan.
Smooth Drug Development offers a full range of clinical trial services from A to Z. These include project management, planning, medical writing, obtaining regulatory and ethical approvals, vendor management, logistics, clinical monitoring, pharmacovigilance, data management, quality assurance and control, biostatistics and final report writing. We offer both stand-alone services and management of the entire clinical trial program.
Our clinical trials team is here to provide you with all the benefits of conducting clinical trials in Uzbekistan while maintaining high quality standards.


